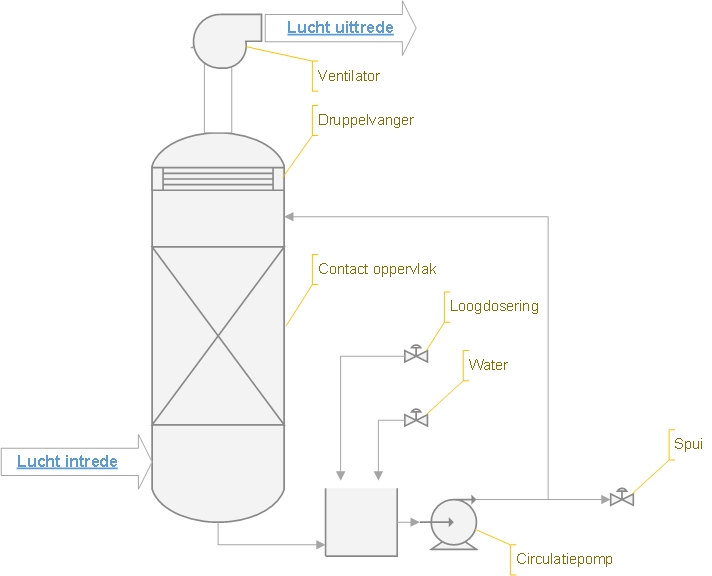
Alkaline scrubber in the chemical industry
Unfortunately, unpleasant odours are also often released in the chemical industry, take chlorine (Cl2) as an example. This is a strongly smelling highly toxic gas and must therefore always be removed from air streams before it can be exhausted to the outside. Chlorine can be absorbed very well in an alkaline scrubber, the chlorine reacts with caustic soda to form sodium chloride or kitchen salt (NaCl) and sodium chlorate (NaClO3).
An alkaline gas scrubber can therefore react acidic components out of an air stream. By adding a base (lye), the pH of the washing water is raised and the acidifying components are neutralized by the lye, whereby salts are formed. The dosing of the base is done by means of a pH control, in most cases the pH value of the washing water varies between 7 and 12.
Depending on the salt formed, more or less washing water is discharged, this is measured on the basis of density and/or conductivity. The drainage can contain up to 15 % of salts and is discharged or evaporated for reuse.
Usually caustic soda (NaOH) is added as a base to the washing water. Often an alkaline scrubber is designed in combination with an odour scrubber. Besides caustic soda, a chlorine solution is dosed to oxidize all odour components. For more information please refer to the odour scrubber
Alkaline scrubber in the chemical industry
Unfortunately, unpleasant odours are also often released in the chemical industry, take chlorine (Cl2) as an example. This is a strongly smelling highly toxic gas and must therefore always be removed from air streams before it can be exhausted to the outside. Chlorine can be absorbed very well in an alkaline scrubber, the chlorine reacts with caustic soda to form sodium chloride or kitchen salt (NaCl) and sodium chlorate (NaClO3).
An alkaline gas scrubber can therefore react acidic components out of an air stream. By adding a base (lye), the pH of the washing water is raised and the acidifying components are neutralized by the lye, whereby salts are formed. The dosing of the base is done by means of a pH control, in most cases the pH value of the washing water varies between 7 and 12.
Depending on the salt formed, more or less washing water is discharged, this is measured on the basis of density and/or conductivity. The drainage can contain up to 15 % of salts and is discharged or evaporated for reuse.
Usually caustic soda (NaOH) is added as a base to the washing water. Often an alkaline scrubber is designed in combination with an odour scrubber. Besides caustic soda, a chlorine solution is dosed to oxidize all odour components. For more information please refer to the odour scrubber.
The advantages of an Air Solutions alkaline scrubber are:
- Completely made of plastic to prevent corrosion
- Relatively compact
- Horizontal and vertical airflow possible
- Very high removal efficiency
- Can be built modular, multi-stage systems
- The formed salts can be reprocessed and reused
Air Solutions has experience with alkaline scrubbers in the following industries:
- MDF wood pressing (formaldehyde)
- Waste treatment plant (hydrogen sulphide H2S)
- Chemical industry (chlorine neutralisation)
- Pharmaceutical industry (phenols and hydrogen fluoride)
In addition to alkaline scrubbers, Air Solutions Holland also offers acid scrubbers, odour scrubbers and plastic chemical storage tanks.
Quality marks:
- ISO 9002
- CE
- KIWA
- VLAREM
- DVS welding certification

DVS

TUV

KIWA

ISO

CE